Cytochromes c are highly conserved proteins found in humans, other eukaryotes, plants, bacteria and Archaea. Their diverse functions and roles in electron transport chains for respiration and photosynthesis are well studied. However, their biogenesis is less well understood, representing a fundamental biological question and the focus of our research. Cytochrome c biogenesis requires covalent heme attachment via two thioether bonds between the heme vinyls and cysteine thiols at a conserved CXXCH motif (Fig. 1A). The requirement for heme attachment makes cytochromes c unique among the cytochromes and is generally agreed to provide high stability and unique properties. Despite their diverse functions and roles in the cell, all cytochromes c are biosynthesized by one of three pathways, termed System I (alpha,
gamma Proteobacteria; plant and protozoal mitochondria; Archaea; Fig. 1B), System II (Gram +; cyanobacteria; chloroplasts; epsilon Proteobacteria; Fig. 1C) and System III (eukaryotic mitochondria, composed of a single enzyme called HCCS). The Sutherland lab focuses on the molecular mechanisms of heme trafficking and attachment in Systems I and II.
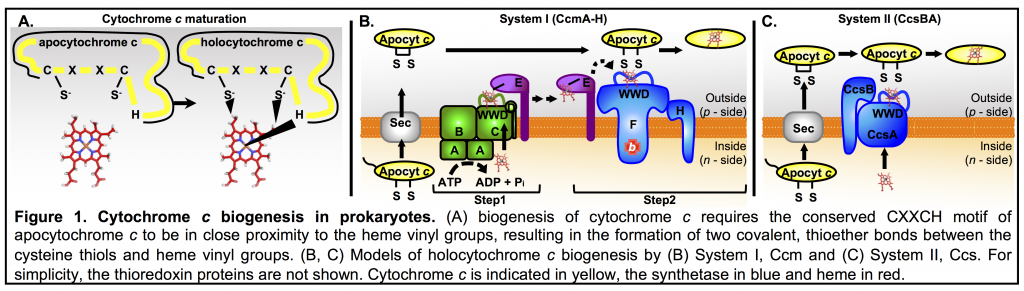
System I: System I is composed of 8 membrane proteins, CcmABCDEFGH, that are proposed to function in two steps. First, heme is transported from the cytoplasm (CcmABCD) to outside the cell and attached to the heme chaperone (CcmE). Then, heme is trafficked to the holocytochrome c synthetase (CcmFH) and covalently attached to cytochrome c (Fig. 1B).
System II: System II is composed of 2 integral membrane proteins, CcsBA, that function to both transport and attach heme to cytochrome c (Fig. 1C).
Biogenesis of cytochromes c is essential for cellular survival and conserved across nearly all organisms. Elucidation of the molecular mechanisms of these pathways is critical to understanding bacterial energetics and survival.
Current Projects
Utilizing a recombinant E. coli system for Systems I and II, these integral membrane proteins are affinity tagged, purify with endogenous heme and are functional, allowing for biochemical and genetic studies of these pathways. The Sutherland Lab focuses on the following fundamental questions:
- How is heme trafficked by these pathways?
- Can heme binding domains be identified?
- What are the requirements for heme attachment to the cytochrome c CXXCH motif?
- How is heme delivered to these pathways?
We are also interested in the cytochrome c biogenesis pathways as a future target for novel antimicrobials:
- What is the role of cytochrome c biogenesis for bacterial survival, particularly in human pathogens?
- Can these fundamental studies lead to novel cytochrome c biogenesis inhibitors?